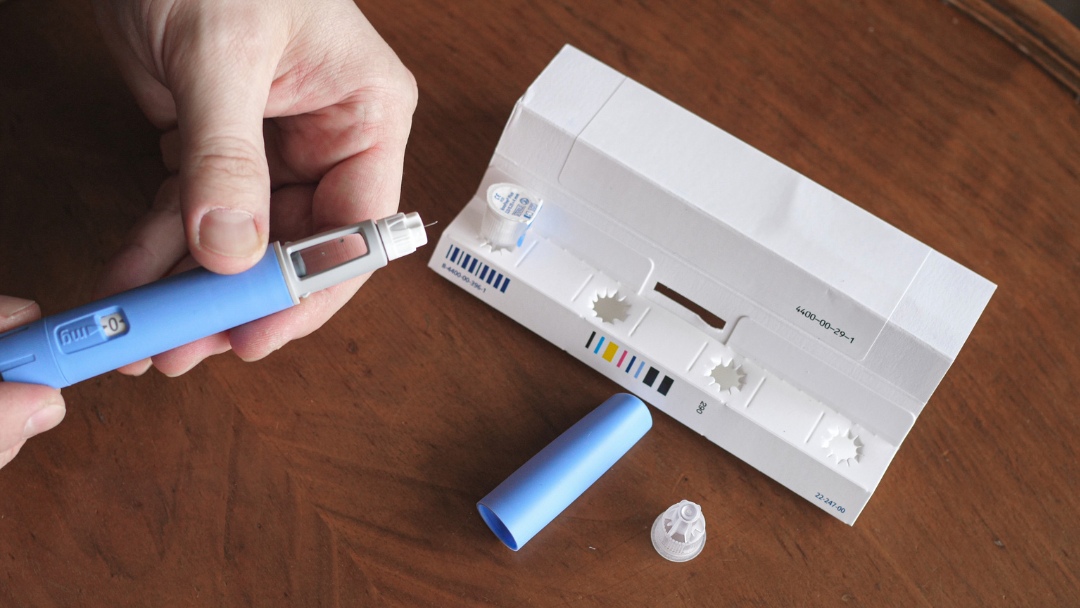
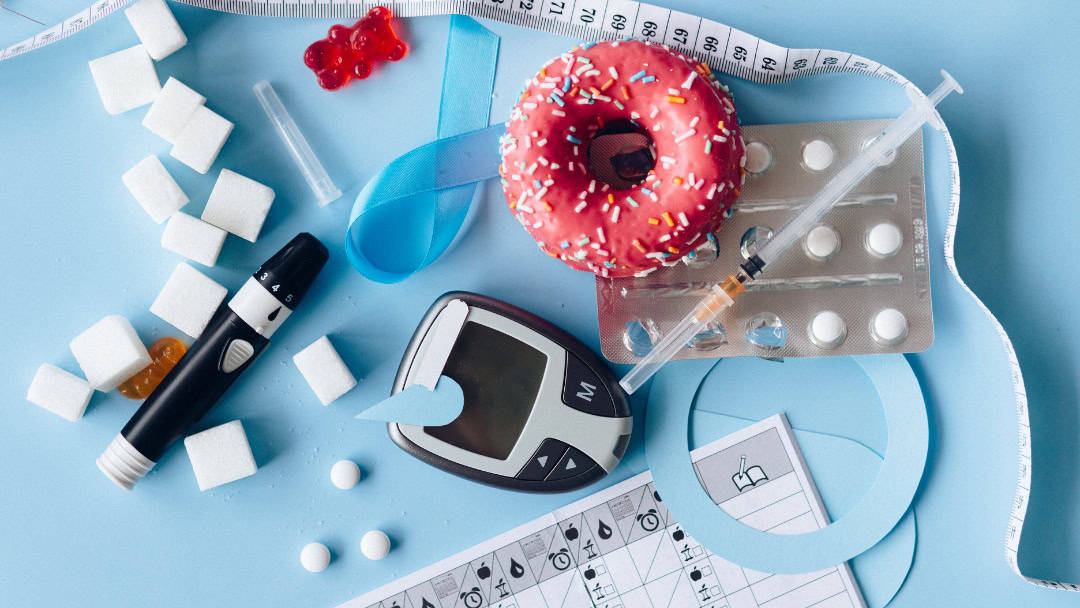
Parents today are being told to trust a medical fix for their child’s weight. In exam rooms across the country, doctors are prescribing injectable GLP-1 drugs to children within minutes of raising weight concerns. Families are reassured these medications are safe, well-studied, and necessary.
But what if the medication intended to protect a child’s future could instead interfere with their development, specifically their brain, hormones, bones and fertility?
Obesity is real, rising, and urgent. One in five U.S. children now meets the criteria for clinical obesity, and 42% of adults are also affected. Children are developing type 2 diabetes in elementary school, and teens face inflammation, depression and severe weight gain.
Action is necessary. But rushing children into a medication with unknown long-term consequences is not the answer.
“We do not know the long-term effectiveness and safety of these medicines in children… there are concerns about pancreas problems, thyroid cancer risk and bone health over the lifetime.”
The root causes of this epidemic are ultra-processed foods, gut dysbiosis, blood-sugar dysregulation, sleep disruption, chronic stress, which are not pharmaceutical issues. They require a different kind of care.
What Are GLP-1 Drugs?
GLP-1 receptor agonists (e.g., Ozempic®, Wegovy®, Mounjaro®) mimic a hormone that reduces appetite and slows digestion. Weight can drop simply because a child eats less, not because their metabolism is repaired.
“These drugs suppress symptoms, they are not correcting the disease process.”
Do GLP-1 Drugs Work for Weight Loss?
Short-term trials (here and here) show weight loss while actively taking the medication. In adolescents, semaglutide did reduce BMI more than lifestyle counseling alone for about a year.
But then what?
When adults discontinue semaglutide, about two-thirds of the weight lost returns within one year often with worsened insulin resistance.
“We are effectively asking children to stay on an experimental drug indefinitely to avoid rapid relapse.”
This implies lifelong dependence when therapy begins in childhood.
What About Hormones, Fertility, Bone Growth and Brain Development?
These drugs have not been studied long-term in youth for:
- Hormone maturation
- Reproductive development
- Bone density and peak bone mass formation
- Neurotransmitter balance and cognition
- Mental health stability under appetite suppression
Animal studies show fetal harm and reproductive toxicity. Emerging reports connect GLP-1 drugs with:
“We must apply the precautionary principle with any medication that affects dopamine and gut-brain signaling during puberty.”
No study has followed treated teens into adulthood.
Serious and Life-Altering Harms Are Already Reported
Regulatory systems are tracking:
- Stomach paralysis (gastroparesis)
- Bowel obstruction, including colon removal
- Acute pancreatitis and kidney failure
- Permanent vision loss
- Sudden deaths under investigation
“These aren’t mild side effects, they are hospitalizing events. We need caution, not hype.”
Thousands of lawsuits cite these harms in real patients.
If GLP-1 Drugs Are Risky, Why Are They Promoted So Heavily in the U.S.?
Pediatric trials are:
- Short in duration
- Small in size
- Funded by the companies seeking approval
Meanwhile, several European systems have slowed adoption.
“Public health services don’t want to pay for [Wegovy] if it won’t tackle underlying health conditions… merely a ‘vanity drug’ will not be covered.”
These drugs are still experimental for obesity, especially in children.
There Is Another Path, and It Works
At 15, Penelope weighed 320 pounds. She was told her biology was broken and that drugs or surgery were inevitable. She believed she had “tried everything.”
But the real root causes had not yet been addressed. When she learned to:
- Remove ultra-processed foods
- Stabilize blood sugar
- Repair gut health
- Regulate her nervous system
- Move daily in ways she enjoyed
She lost 160 pounds naturally, and has kept it off for more than a decade. Her biology wasn’t special. Her inputs changed.
This is not rare. It is repeatable when children are given the right support.
“Our first responsibility is to protect a developing child’s future potential.”
What Parents Should Ask Before Considering GLP-1 Treatment
- What is the exit plan?
- How will development be protected?
- Has a true metabolic assessment been done?
- Have root-cause lifestyle strategies truly been exhausted?
Children deserve healing, not dependency.
We Are Listening
If your family has experienced a severe adverse event from a GLP-1 medication or if you are a clinician witnessing harms, Documenting Hope is collecting reports confidentially. Your voice can help protect millions of children.
About Helene Leeds MS and Penelope Popken
Helene Leeds, MS is a leading expert in lifestyle medicine who has spent 30+ years helping families overcome obesity and chronic disease naturally. She has trained over 1,000 health coaches worldwide and developed programs used by top medical institutions. As a MasterChef finalist and media contributor featured in Forbes and FOX TV, she blends science with real-world solutions that protect a child’s long-term health.
Helene is here to help families navigate evidence, avoid harm, and access proven root-cause care. If you have questions or a story to share, she would love to hear from you.

Penelope Popken is a wellness leader, speaker, and co-founder of Step It Up and MAHA Girls. At 15 years old, she weighed 320 pounds and was struggling with anxiety, depression, and metabolic illness. Instead of accepting drugs or surgery as her only options, she turned to whole foods and holistic healing, losing 160 pounds naturally and transforming her physical and mental health.
Her mission now is to help women break free from unhealthy cycles, rebuild their confidence, and reclaim their energy. Through her programs and advocacy, Penelope empowers women across America to choose healing, strength, and a life they love.
Still Looking for Answers?
Visit the Documenting Hope Practitioner Directory to find a practitioner near you.
Join us inside our online membership community for parents, Healing Together, where you’ll find even more healing resources, expert guidance, and a community to support you every step of your child’s healing journey.
Sources & References
Chen, W., et al. Psychiatric adverse events associated with GLP-1 receptor agonists: a real-world pharmacovigilance study based on the FDA Adverse Event Reporting System database. Front Endocrinol (Lausanne). 2024 Feb 6:15:1330936.
Fitch, A., et al. Application of nutrition interventions with GLP-1 based therapies: A narrative review of the challenges and solutions. Obes Pillars. 2025 Aug 28:16:100205.
Hales, C.M., et al. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief. 2020 Feb:(360):1-8.
Hall, K.D., et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019 Jul 2;30(1):67-77.e3.
Huang, L., et al. Pancreatitis associated with immune checkpoint inhibitors: a pharmacovigilance analysis based on FDA adverse event reporting system (FAERS) database. Front Pharmacol. 2025 Sep 22:16:1635372.
Hunt, J.E., et al. GLP-1 and Intestinal Diseases. Biomedicines. 2021 Apr 5;9(4):383.
Jastreboff, A.N., et al. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022 Jul 21;387(3):205-216.
Kompaniyets, L., et al. Prescriptions for Obesity Medications Among Adolescents Aged 12-17 Years with Obesity – United States, 2018-2023. MMWR Morb Mortal Wkly Rep. 2025 Jun 5;74(20):337-344.
Lu, W., et al. Neuropsychiatric adverse events associated with Glucagon-like peptide-1 receptor agonists: a pharmacovigilance analysis of the FDA Adverse Event Reporting System database. Eur Psychiatry. 2025 Feb 4;68(1):e20.
Mozaffarian, D., et al. Nutritional priorities to support GLP-1 therapy for obesity: A joint Advisory from the American College of Lifestyle Medicine, the American Society for Nutrition, the Obesity Medicine Association, and The Obesity Society. Obesity (Silver Spring). 2025 Aug;33(8):1475-1503.
Peterseim, C.M., et al. Metabolic Syndrome: An Updated Review on Diagnosis and Treatment for Primary Care Clinicians. J Prim Care Community Health. 2024 Jan-Dec:15:21501319241309168.
Quarenghi, M., et al. Weight Regain After Liraglutide, Semaglutide or Tirzepatide Interruption: A Narrative Review of Randomized Studies. J Clin Med. 2025 May 28;14(11):3791.
Ramsey, D.J., et al. GLP-1 Receptor Agonists and Sight-Threatening Ophthalmic Complications in Patients With Type 2 Diabetes. JAMA Netw Open. 2025 Aug 1;8(8):e2526321.
Rubino, D., et al. Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA. 2021 Apr 13;325(14):1414-1425.
Sills, E.S., et al. Semaglutide and human reproduction: caution at the intersection of energy balance, ovarian function, and follicular development. Reprod Biol Endocrinol. 2025 Aug 8;23(1):116.
Wang, M., et al. Exploring potential associations between GLP-1RAs and depressive disorders: a pharmacovigilance study based on FAERS and VigiBase data. EClinicalMedicine. 2025 Jul 29:86:103385.
Weghuber, D., et al. Once-Weekly Semaglutide in Adolescents with Obesity. N Engl J Med. 2022 Dec 15;387(24):2245-2257.
West, S., et al. Weight regain following the cessation of GLP-1 RAs for weight management: a systematic review and meta-analysis. University of Oxford, Nuffield Department of Primary Care Health Sciences. 2025.
Resources
Articles
Boots, Christina E. ARE GLP-1S THE NEWEST FERTILITY TREATMENT? Northwestern Medicine. Nov 2024.
Collier, Lorna. New Weight-Loss Drugs Will cost, shortages and payment debates hamstring access? CQ Press. 7 Jun 2024.
EMA statement on ongoing review of GLP-1 receptor agonists. European Medicines Agency. 11 Jul 2023.
Janković, Saša. More than 500 people hospitalised following use of weight-loss drugs, says health minister. The Pharmaceutical Journal. 12 Mar 2025.
January – March 2024 | Potential Signals of Serious Risks/New Safety Information Identified by the FDA Adverse Event Reporting System (FAERS). U.S. Food & Drug Administration. 3 Jul 2024.
Langel, Stephen. ‘Sweet tooth gene’ might help determine who benefits from weight loss drugs. Ideastream Public Media. 29 Sep 2025.
Obesity: Childhood Obesity Facts. U.S. Centers for Disease Control. Accessed 6 Nov 2025.
Obesity and overweight. World Health Organization. 7 May 2025.
Update on FDA’s ongoing evaluation of reports of suicidal thoughts or actions in patients taking a certain type of medicines approved for type 2 diabetes and obesity. U.S. Food & Drug Administration. 11 Jan 2024.